How are the results interpreted and what do they mean?
Specific circulating tumor cell (CTC) cutoffs indicating favorable vs unfavorable prognosis for metastatic breast (mBC), prostate* (mPC), and colorectal (mCRC) cancers were determined through comprehensive analysis to determine correlation between disease progression and the lowest number of CTCs within a preset range across multiple test subjects.
CTCs Above or Below a Predetermined
Cutoff Number Predict Prognosis
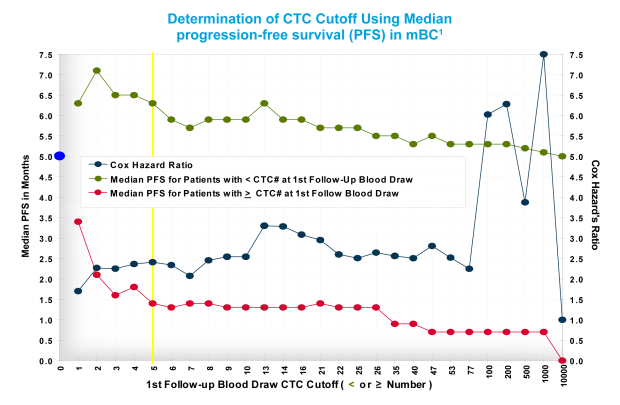
For example, in determining the CTC cutoffs for mBC, 90 patients were stratified into 2 groups: positive (having ≥ the CTC cutoff) and negative (having < the CTC cutoff). For every possible cutoff from 1 to 82 CTC, and at selected cutoffs up to 10,000 CTCs, the median PFS was calculated for the positive and negative patient groups. For the entire population, the median PFS was 5.0 months (indicated by the blue dot on the y axis).1
In the positive group, the median survival in patients with a cutoff of 1 CTC was 3.5 months, whereas at a cutoff of 2 or 3 CTCs, the PFS declined sharply to 2.0 and 1.5 months, respectively. At a cutoff of 5 or more CTCs, the decline in PFS plateaued, ranging between approximately 1.4 to 0.6 with CTCs up to 1000. Thus, ≥5 CTCs was determined to be the CTC cutoff for unfavorable prognosis.1
Likewise, in the negative group, the median survival in patients with a cutoff of <5 CTCs had a significantly longer PFS (approximately 6.3-7.1 months), whereas PFS began to drop, then plateaued at a cutoff of <5 CTCs. Thus, <5 CTCs was determined to be the cutoff for favorable prognosis.1 Similar methodologies were used to determine CTC cutoffs for mPC and mCRC.
Tumor Cell Cutoffs for mBC, mPC*, and mCRC

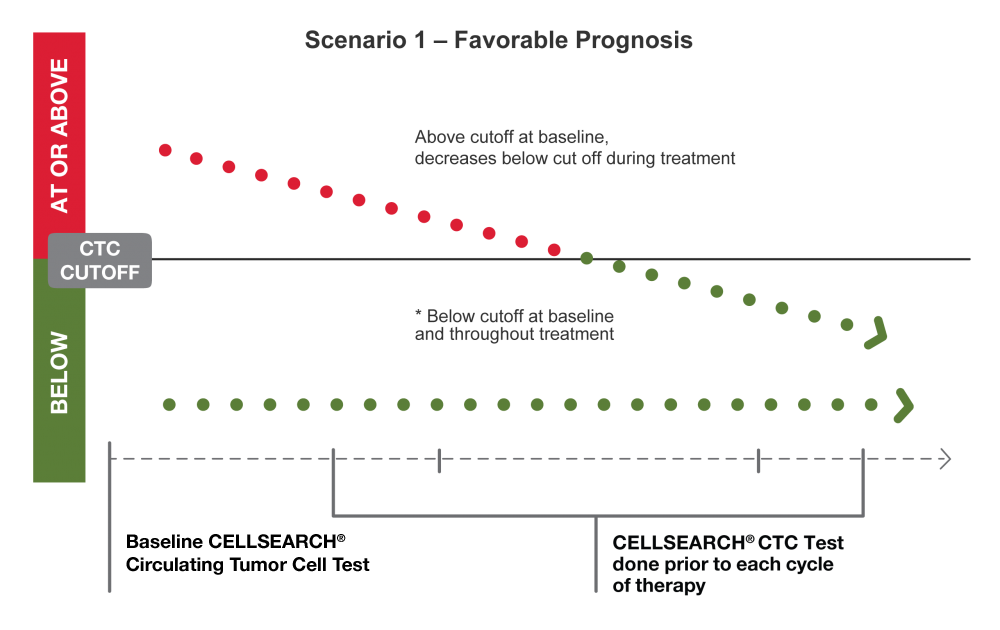
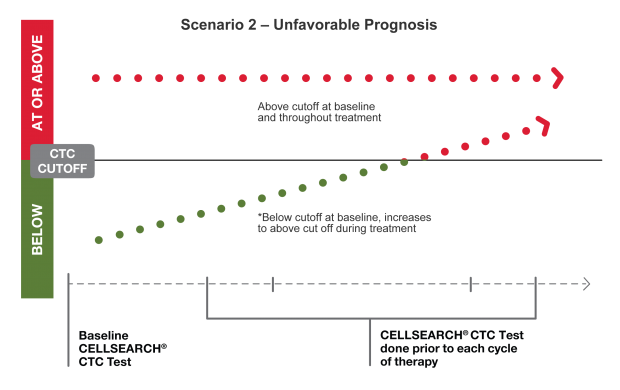
Clinical studies have demonstrated the predictive accuracy of serial CTC monitoring in determining prognosis at any time during the course of disease.2-4 CTCs, along with prognosis, can shift over time, which can inform your future decisions.
*Metastatic prostate cancer patients were defined as having two consecutive increases in the serum marker prostate-specific antigen above a reference level, despite standard hormonal management. These patients are commonly described as having androgen-independent, hormone-resistant, or castration-resistant prostate cancer. For more information on the intended use and limitations for the CELLSEARCH® Circulating Tumor Cell Test, please refer to the Instructions for Use which can be found at documents.cellsearchctc.com.
References:
- K-3 Letter Response Clinical Section; data on file. Menarini Silicon Biosystems Inc.
- Cohen SJ, Punt CJA, Iannotti N, et al. J Clin Oncol. 2008;26(19):3213-3221.
- Cristofanilli M, Hayes DF, Budd GT, et al. J Clin Oncol. 2005;23(7):1420-1430.
- de Bono JS, Scher HI, Montgomery RB, et al. Clin Cancer Res. 2008;14(19):6302-6309.