The Clinical Relevance of the CELLSEARCH® Circulating Tumor Cell Test
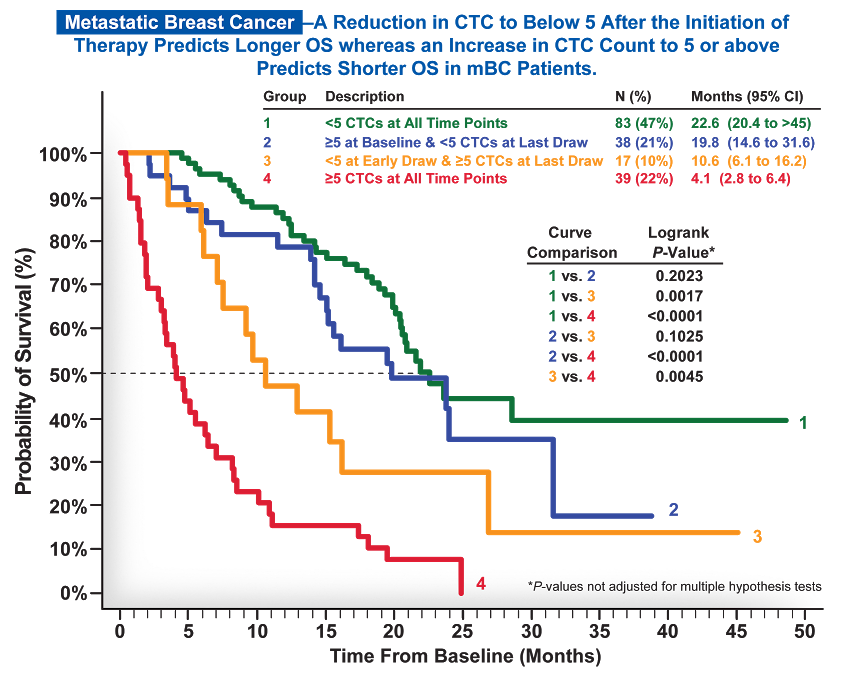
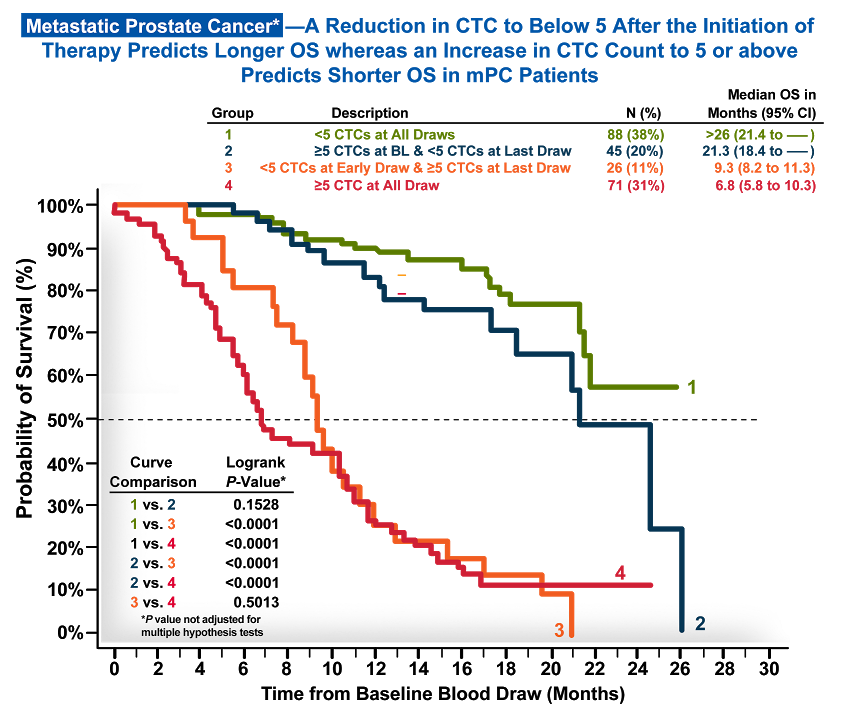
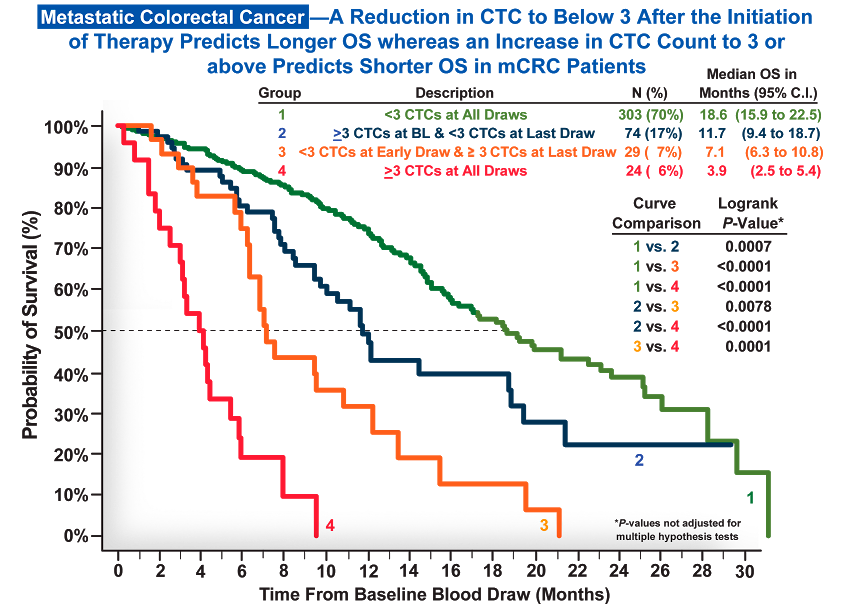
Circulating tumor cells (CTCs), as measured by the CELLSEARCH® CTC Test, are a strong, independent predictor of overall and progression-free survival in metastatic breast, prostate*, and colorectal cancer.1-3
Throughout therapy, CELLSEARCH® CTC testing can be used to monitor a patient’s status by showing you whether their prognosis on any given therapy is favorable or unfavorable at any given time.
*Metastatic prostate cancer patients were defined as having two consecutive increases in the serum marker prostate-specific antigen above a reference level, despite standard hormonal management. These patients are commonly described as having androgen-independent, hormone-resistant, or castration-resistant prostate cancer. For more information on the intended use and limitations for the CELLSEARCH® Circulating Tumor Cell Test, please refer to the Instructions for Use which can be found at documents.cellsearchctc.com.
References:
- CELLSEARCH® Circulating Tumor Cell Kit (Epithelial) Instructions for Use.
- de Bono JS, Scher HI, Montgomery RB, et al. Clin Cancer Res. 2008;14(19):6302-6309.
- Clinical Trial data for the CELLSEARCH® Circulating Tumor Cell Test in mPC; data on file. Menarini Silicon Biosystems Inc.